Plan Design and Development
Plan Design and Development
(Pharmaceutical APIs and/or Pharmaceutical Formulations compliance to WHO-GMP/PICS/EUGMP/UK MHRA)
(Medical Devices and/or In Vitro Diagnostics compliance to MDR 2017/ EU MDR 745/746 and US 21CFR 820)
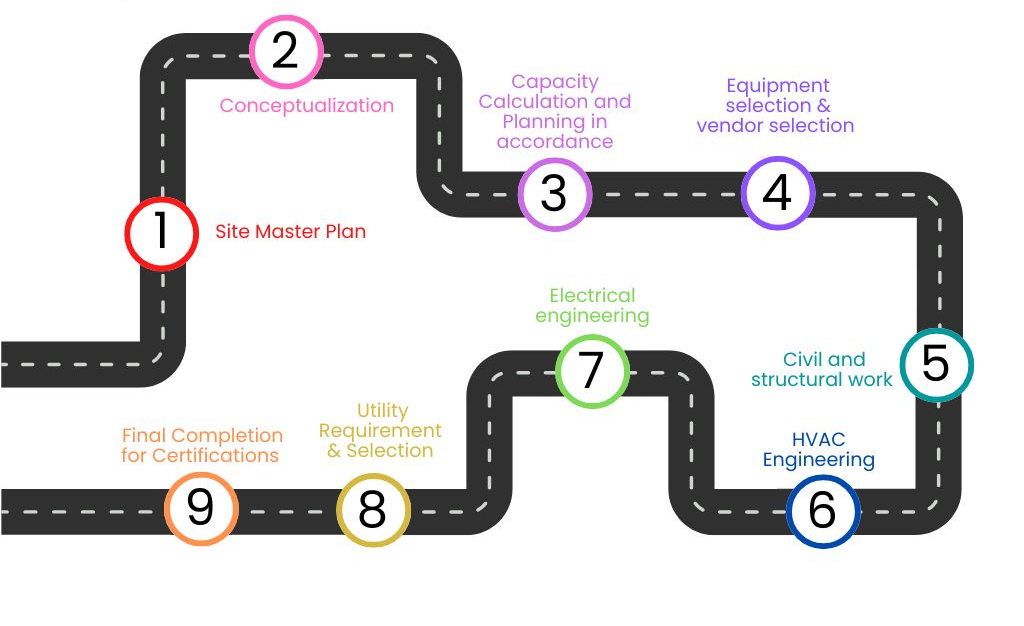
Plant Designing
This process ensures a structured approach to facility planning, from initial Site Master Planning and Conceptualization to Capacity Calculation, Equipment & Vendor Selection, and Infrastructure Development. It covers key engineering aspects such as Civil, Structural, HVAC, Electrical, and Utility Systems, ensuring seamless integration. The final stage focuses on Regulatory Compliance and Certification, ensuring the facility meets industry standards for operational readiness.
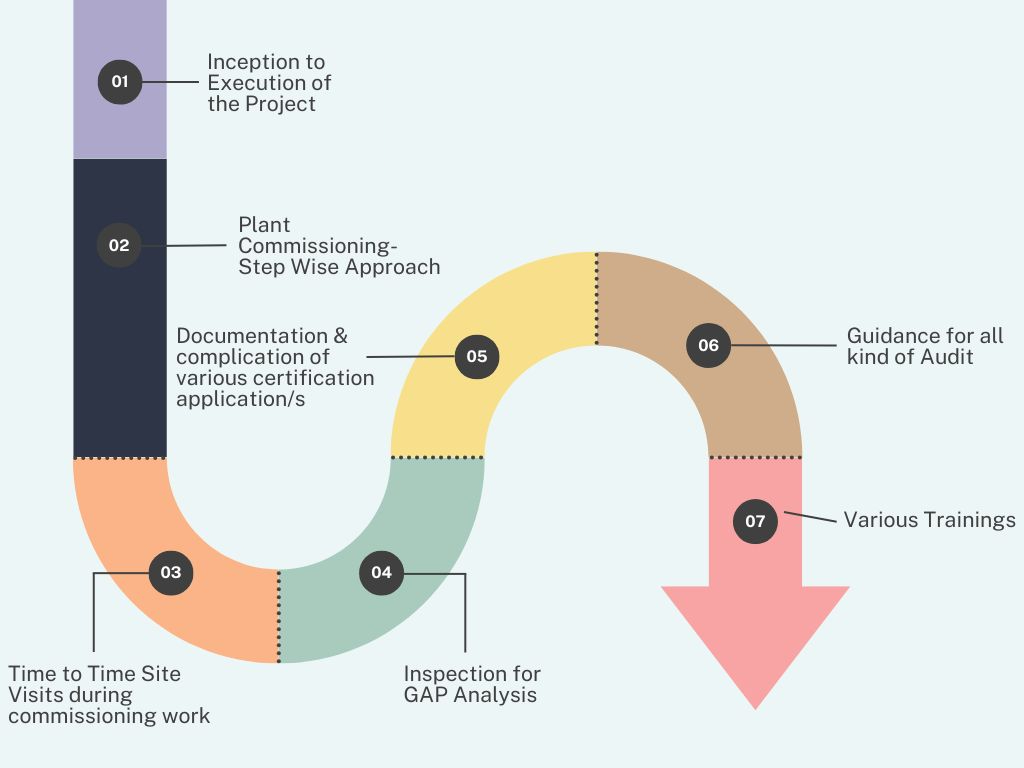
Commissioning Work
This process ensures a smooth transition from project inception to plant commissioning through a step-wise approach, including site visits, GAP analysis, certification documentation, audit guidance, and training programs. It ensures regulatory compliance, operational efficiency, and successful project execution.
Know more services
Pharmaceuticals formulation and Active Pharmaceuticals Industries
Various Regulatory Services concerning to the State Licensing Authorities (SLA)
Various Regulatory Services concerning to the Central’s Zonal Licensing Authorities (CLA-Zonal CDSCO)
Medical Devices and In-Vitro Diagnostics
Applications for Manufacturing Test license under Form MD 12 (Medical Devices/IVDs) – CDSCO
Applications for Manufacturing License under Form MD 9 (Medical Devices/IVDs) – CDSCO
Cosmetics
Applications for Manufacturing License under COS 5
Applications for Additional Product Approvals



